Sponsored Content
The Dark Side of Imitation – Counterfeit Alcohol and the Fight for Safety
The Transported Asset Protection Association (TAPA) has published a white paper on the scourge of counterfeit alcohol in response to a string of high-profile incidents that have claimed hundreds of lives.
The document details some of the most notorious incidents in recent years and explains how a brand protection standard (BPS) developed by TAPA Asia Pacific (TAPA APAC) can strengthen the supply chain against counterfeit goods by encouraging logistics companies to "design out" vulnerabilities. Read more
Sponsored Content
The Dark Side of Imitation – Counterfeit Alcohol and the Fight for Safety
The Transported Asset Protection Association (TAPA) has published a white paper on the scourge of counterfeit alcohol in response to a string of high-profile incidents that have claimed hundreds of lives.
The document details some of the most notorious incidents in recent years and explains how a brand protection standard (BPS) developed by TAPA Asia Pacific (TAPA APAC) can strengthen the supply chain against counterfeit goods by encouraging logistics companies to "design out" vulnerabilities. Read more
Press Releases
-
》 Antares Vision Group and Spherity expand partnership with reseller agreement to deliver DSCSA ATP credentials
-
》 TraceLink customers demonstrate readiness as DSCSA dispenser deadline arrives
-
》 Manufacturo and SolarFlexes partner to modernize utility-scale solar hardware production and traceability
-
》 4th Forum Unique Codes – Securikett brings experts together to discuss the security of the Digital Product Passport
Partners




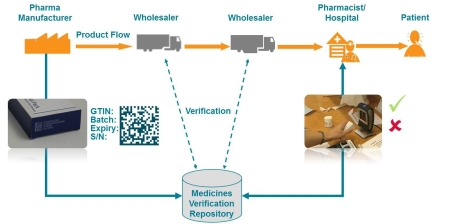
 The European Federation of
Pharmaceutical Industries & Associations (EFPIA) has laid out
its vision of how the system of safety features enshrined in the EU
Falsified Medicines Directive (FMD) might work in a call-for-tender
document.
The European Federation of
Pharmaceutical Industries & Associations (EFPIA) has laid out
its vision of how the system of safety features enshrined in the EU
Falsified Medicines Directive (FMD) might work in a call-for-tender
document.