
The EU Falsified Medicines Directive (FMD) and related Delegated Acts on Safety Features outlines complex new track and trace regulations that will begin phasing in February 2019 and apply to those who manufacture, sell, or dispense medications in the EU and certain other European countries.
To ensure supply to one of the largest global markets, now is the time for pharmaceutical companies, their CMO/CPO partners and their 3PL partners to fully understand the extensive serialization, compliance reporting, verification and other requirements they will be held accountable for, in order to ensure full FMD compliance.
This article will highlight key requirements of the new track and trace regulations of the FMD, some of the unique challenges facing companies seeking to build a secure and flexible compliance infrastructure, and initial learnings as companies have started to implement their FMD compliance program.
The FMD and its track and trace model
The FMD introduces stringent regulations aimed to improve public health with new harmonized, pan-European measures that control and monitor the trade pathway of medicines to safeguard them for human use. The FMD track and trace regulations include multiple, diverse rules for all stages of the product, distribution and dispensation lifecycle, which derive from a strong foundation of safety feature regulations and rigorous verifications.
Fundamentally, the FMD enables a point of dispense verification model for medicines. To enable this model, the law requires the implementation of several different track and trace capabilities. We'll touch on each of the three core regulations here.
#1: Serialization
The FMD requires serialization at the saleable pack or secondary level. For each pack of drug product, a unique serial number, coupled with the manufacturer product code, batch number and expiration date, are to be encoded in both a GS1 2D datamatrix and in human-readable form. A fifth data element, such as a national reimbursement number, may be required based on country requirements.
#2: Compliance reporting
The Marketing Authorization Holder (MAH) has several reporting and notification requirements under the FMD. The primary regulations apply to product master data and serialized product pack data. First, master data about the product, including product codes, form, strength, doses per pack, pack type, and target market(s) for distribution, must be reported to the European hub for each unique product form produced along with any subsequent updates. Serialized product pack data must also be reported, including product codes, lot/batch number, expiry data and serial numbers, for each unit of drug product shipped into the supply chain. Drug product status must also be maintained and updates made to the EU hub. These status updates may be required at a batch level if a recall is initiated, or at a saleable unit level in situations such as the decommissioning of serial numbers due to destruction of drug product.
#3: Verification and safety features
The FMD requires verification of the safety features, including the serialized product identifier, at least once before the product leaves the supply chain and is dispensed to the patient. The drug package barcode is scanned, and the information is submitted in a query to a national repository system which contains data about the drug package that was originally submitted by the manufacturer or marketing authorization holder. The national repository checks to verify that the product code and serial number of the product scanned matches an active unique identifier in the system. Under simple distribution when the product moves from MAH to wholesaler to pharmacy, this verification is performed by the pharmacy dispenser.
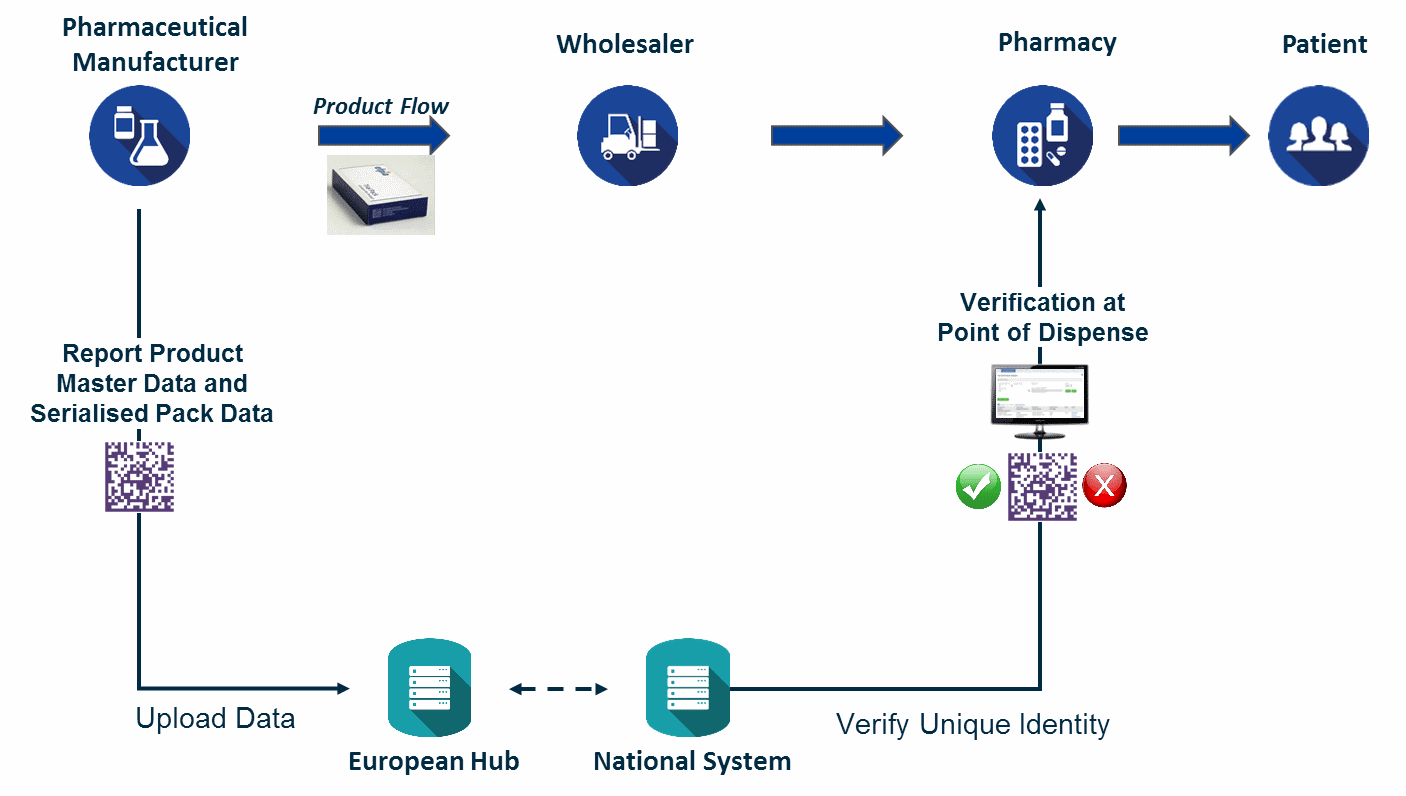
The regulations provide for a myriad of additional verification checks, reports and notifications depending on product path and changes in packaging. Should one wholesaler purchase product from another wholesaler, they must verify the safety features of the purchased product prior to resale. Saleable product returns also trigger verification inquiries prior to resale. In addition, if parallel importation and repackaging occurs of original manufactured product, a cascade of verification inquiries, decommissioning status updates, product master data reports and product pack/serialization data reports are required against the original product and the repackaged product.
Simple in concept, complex in implementation
The FMD creates an umbrella harmonized regulation covering the member states of the EU, plus four other countries (Norway, Iceland, Liechtenstein and Switzerland). The law also provides flexibility in how the regulations apply for drug products targeted for dispensation within a given country. So, a pharmaceutical company preparing for FMD regulations needs to design their serialization and compliance infrastructure both for the extreme scalability challenges presented by the FMD and the flexibility required to serve the member states. For example, a drug product may be regulated as a prescription medicine in one member state but not another, thereby creating serialization requirements for some drug packages and not others.
Certain prescription drug products may be white-listed, or exempted, from the safety feature requirements. A member state may follow the standard GS1 GTIN to identify the drug product, or they may require a unique national product code to be used. Member states may also require additional data to be captured, stored and reported with each drug product, such as a national reimbursement number. These are some of the complexities facing a pharmaceutical company preparing their internal packaging sites and external CMO network, and the CMO looking to serve a diverse pharma client base when preparing for FMD compliance.
Managing the network and scalability challenges
Implementing a secure compliance infrastructure for EU FMD means that pharmaceutical companies and their supply network partners are challenged with mastering a never-before-seen level of network management and system scalability.
A mid-sized pharma company may use a dozen or more internal packaging sites and external contract partners to produce their serialized products for markets in the EU and globally. The disparate packaging sites' diverse serialization line management systems need to be integrated across multiple transactions, exchanging serialized packaging information and related data at the individual package level when triggered by numerous operational events. The challenges continue as the serialized product moves through the internal distribution network. Product pack data, including related serialization information, needs to be reported for medicines shipped into the supply chain. So, internal distribution centers and 3PLs must be integrated into the compliance infrastructure to ensure that appropriate operational triggers and serialization data exchanges are enabled for compliance reporting.
FMD compliance creates significant data management and transaction processing challenges for all stakeholders in the supply chain as they prepare for serialization, compliance reporting and verification of product identity. Medications in Europe are generally packaged and sold at the “unit of use” level, so the unit volume of product that needs to be serialized can range from the tens of thousands into the hundreds of millions of units per year for a given company. The shift from volumetric, lot-level management of production orders to serialized product tracking at the unit level creates an explosion of transactions and associated compliance data flowing between systems controlling serialization management, packaging lines and distribution centers.
Management of uniquely serialized product regulated under the FMD will generate 1,000 times the data storage requirements and 10,000 times the transaction volumes of those seen today for lot-level product.
Even for smaller specialty pharmaceutical companies and virtual life sciences organizations with less unit volume and supply networks, this data explosion and increase of transaction events will be problematic, as their existing infrastructures weren't designed for this magnitude of data processing and storage, and the resources (human, capital) available to handle this unexpected growth are typically limited.
Overall, the universe of data to be produced, managed, and reported upon will be massive and the synchronized network of systems, facilities and partners will be larger than ever before. Preparations to manage network and scale requirements must also incorporate flexibility as data and events will vary based on target markets falling under the EU FMD and the diverse global regulations that a multi-national company will face in the US, South Korea, India, Brazil, Russia and other countries that do not align to those in the EU. While regulatory preparation is necessary, it is not sufficient enough to provide a secure and flexible serialization infrastructure, as one must also account for the requirements imposed by the supply network.
Operational and trade partner requirements for serialisation planning
EU FMD establishes a fairly clear and comprehensive set of regulations and implementation requirements for compliance by February 2019. That said, this is not the end of the challenges facing pharmaceutical companies and their supply partners. As we have seen across multiple other track-and-trace regulations, from US DSCSA to South Korea, business operations and trade partners often impose additional data management and operational requirements in addition to the hard regulations.
Serialization at other packaging levels, such as at the transport case level, is not required nor is aggregation required under EU FMD regulations. Regardless, considerable industry discussion has already started on the potential business or operational needs for multi-level serialization and related aggregation activities. For example, as products move through internal distribution facilities or 3PL locations, the need to know exactly which drug packages and their unique identity have been shipped into the supply chain is critical. With individual units of drug product packaged into sealed cases or transported on pallets, it creates the challenge of being able to efficiently tell which units were shipped and should be reported upon without resorting to breaking down pallets and opening cases to scan individual packages. To resolve this issue, logistics leaders are now looking at how to acquire serialization data, including aggregation information, to help facilitate their operations during receiving, pick, pack and ship activities as well as for saleable returns handling.
How can I prepare now for the 2019 deadline?
With the publication of the final Delegated Acts and a closer look at the many nuanced operational and data management requirements, it's clear that a highly complex set of product information, serialization data and serialized product events need to be managed and exchanged with supply partners to enable a secure, scalable, and cost-effective EU compliance infrastructure.
In order to meet the EU FMD requirements by the February 2019 deadline, the thousands of pharmaceutical companies, CMOs, 3PLs and parallel importers serving the EU market have a lot of work ahead of them to ensure serialization readiness at a massive scale and full compliance readiness for a complex set of reporting requirements.
By starting now, you can develop a clear strategy for understanding the data you need to collect and manage, the network you need to exchange information with, and the serialization management and compliance reporting tools that will ensure you reduce time, cost and risk in meeting FMD requirements. February 2019 may seem far away, but as experience across the United States, India, South Korea, China and Brazil has shown, serialization and compliance readiness always takes much longer and is much more complicated than expected. The time to prepare for EU compliance is now.
Brian Daleiden is vice president of industry marketing at TraceLink.
©
SecuringIndustry.com






